Abstract
Background: Selinexor, an exportin 1 (CRM1/XPO1) inhibitor, has demonstrated anti-leukemic effects as a single agent and in combination with anthracyclines and DNA damaging agents. HiDAC/Mito is an effective induction regimen for patients with relapsed/refractory (R/R) AML and has a reported overall response rate (ORR) of 55% at our institution. We hypothesized that adding selinexor to HiDAC/Mito would be feasible and have synergistic anti-leukemic effects. Early results of the trial were previously reported (Wang et al., J Hematol Oncol, 2018), and here we present more mature data on survival and relapse.
Methods: We performed a phase 1 dose escalation trial with cohort expansion in patients with AML. This study tested increasing doses of selinexor combined with age-adjusted HiDAC/Mito (NCT02573363). The primary endpoint was to determine the maximum tolerated dose of the regimen. Selinexor was given orally on days 2, 4, 9, and 11 during the induction phase. HiDAC (1.5 to 3 g/m2 depending on age, IV over 3 hours) followed immediately by Mito (20 to 30 mg/m2 IV over 1 hour) was administered on day 1 and 5. Initial selinexor dose was 60mg (~35mg/m2) followed by a dose escalation to a target level of 80mg (~50mg/m2). Patients who entered remission proceeded to stem cell transplantation (SCT) or consolidation chemotherapy with HiDAC/selinexor followed by maintenance therapy with weekly selinexor alone for up to one year. Dose limiting toxicity (DLT) were only evaluated during dose escalation and was defined as any grade 3 or greater non-hematologic toxicity, except transient (<48 hours) nausea/vomiting or liver function abnormalities, or by persistent bone marrow aplasia lasting >56 days in the absence of disease. Once a dose level was declared tolerable, more patients could be enrolled at that level to provide additional safety, tolerability, and efficacy data.
Results: The study enrolled a total of 28 patients from October 2015 to October 2017. Selinexor dose levels were 60mg (n=3) and 80mg (n=25). Median age = 61 (range 37 - 76). De novo AML = 15 (54%); secondary AML = 12 (43%), and therapy-related AML = 1 (3%). Of these, 13 patients had R/R disease (6 primary refractory, 6 in first relapse, 1 in second relapse). Fifteen (54%) patients were previously untreated. Molecular/genetic subgroup profiles by European Leukemia Net 2010 criteria included favorable = 6 (21%), intermediate I = 9 (32%), intermediate II = 5 (18%), and adverse = 8 (29%).
No DLTs were observed in dose escalation. Myelosuppression was universal. Median time to count recovery (ANC >1.0 x 109/L, platelet count >100 x 109/L for the 16 responding patients was 46 days. Febrile neutropenia occurred in 21 (75%) patients. Common selinexor-related adverse effects included diarrhea (32%), electrolyte disturbances (32%), bacteremia (32%), anorexia (29%), nausea/vomiting (29%), fatigue (25%), and acute kidney injury (25%). One patient from the expansion cohort died from hemorrhagic stroke prior to completing induction.
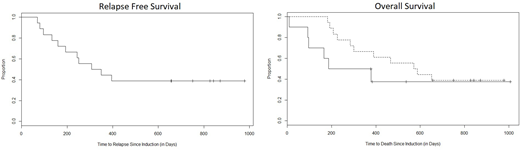
The ORR was 64% (18/28 pts): complete remission (CR) = 46% (13 pts), CR with incomplete count recovery (CRi) = 14% (4 pts), partial remission (PR) = 4% (1 pt), and treatment failure (TF) = 36% (9 pts). ORR was 87% (9 CR, 3 CRi, 1 PR, 2 TF) for newly diagnosed pts and 38% (4 CR, 1 CRi, 8 TF)) for R/R pts. Of the responders, 6 proceeded to consolidation without allo-SCT, 10 eventually underwent allo-SCT, and 2 relapsed prior to either. The 10 non-responding patients proceeded to another line of therapy, and 3 eventually underwent allo-SCT. Eleven (40%) patients are alive with a median observation period of 13 months (range 8 days to 34 months). The median relapse free survival (RFS) and overall survival (OS) was 11 and 16 months, respectively. The 1-year PFS and OS was 44% and 61%, respectively. Median OS was 9 months for non-responders and 19 months for responders (HR 1.8, 95% CI 0.6 - 5.7, p=0.2); 1-year OS rates were 50% vs 67%, respectively. One CR patient completed consolidation and maintenance without allo-SCT remains in remission 33 months later.
Conclusions: The selinexor/HiDAC/Mito regimen is feasible and tolerable at selinexor doses up to 80mg/day or ~50 mg/m2/day twice weekly. This regimen yields an ORR of 64% based on currently available data. We had previously reported molecular correlatives demonstrating the effect of selinexor. The recommended phase 2 dose is 80mg of selinexor.
Larson:Ariad/Takeda: Consultancy, Research Funding; BristolMyers Squibb: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding. Odenike:Agios: Research Funding; Astex: Research Funding; Dava Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI/Baxalta: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Oncotherapy Science: Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; NS Pharma: Research Funding; Celgene: Research Funding; Gilead Sciences: Research Funding; ABBVIE: Honoraria, Research Funding; Janssen: Research Funding. Bishop:United Healthcare: Employment; Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Juneau Therapeutics: Speakers Bureau; Celgene: Honoraria, Speakers Bureau; Novartis Pharmaceuticals Corporation: Speakers Bureau. Curran:Merck: Research Funding. Stock:Jazz Pharmaceuticals: Consultancy. Liu:BMS: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal